Are you among those interested in knowing what Casgevy is? Stay with me as we explore in detail, everything you ought to know about therapy.
Casgevy is manufactured by Vertex Pharmaceuticals Incorporated, it is known generically as exagamglogene autotemcel (or exa-cel), a gene therapy used to treat sickle cell disease (SCD) in patients 12 years and older who have recurrent vaso-occlusive crises (VOCs).
VOCs occur when sickled red blood cells block blood flow, depriving tissues of oxygen. This therapy is notable because it’s administered as a one-time intravenous suspension.
Casgevy employs CRISPR/Cas9 technology to edit specific parts of the DNA containing the BCL11A gene.
This gene plays a role in the production of fetal hemoglobin (HbF) and adult hemoglobin (HbA).
By reducing the activity of the BCL11A gene, the therapy helps patients continue to produce more HbF and less HbA, which in turn decreases the production of sickle-shaped red blood cells.
Read Also; Hydmoxia capsules: uses, dosage,side effects and composition
The treatment involves harvesting the patient’s own hematopoietic stem and progenitor cells, editing them using CRISPR/Cas9 technology, and then infusing the edited cells back into the patient as part of an autologous hematopoietic stem cell transplant.
What are progenitor cells?
Progenitor cells are a type of cell found in the body that have a specific and limited capacity to divide and differentiate.
They are derived from stem cells but are further along the path toward becoming a specialized cell type.
Understanding and harnessing the capabilities of progenitor cells is of great interest in regenerative medicine and tissue engineering.
They have potential applications in treating a variety of diseases and injuries by replacing damaged or diseased cells.
They are a focus of research for potential therapeutic uses, including in gene therapy and regenerative medicine, where they could be used to replace or repair damaged tissues.
Here are some key points about progenitor cells:
Limited Differentiation
Unlike stem cells, which can potentially differentiate into many different cell types and self-renew indefinitely, progenitor cells are often committed to differentiating into a particular cell type or a limited range of cell types.
Role in Tissue Maintenance and Repair
Progenitor cells play a crucial role in the maintenance, repair, and regeneration of tissues.
They are particularly important in tissues that require constant renewal, such as the skin, blood, and lining of the gut.
Intermediate State
They are often considered to be in an intermediate state between stem cells and fully differentiated cells. Progenitor cells typically have less capacity for self-renewal than stem cells.
Types of Progenitor Cells
There are various types of progenitor cells, depending on the tissue they are found in.
For example, in the blood system, hematopoietic progenitor cells give rise to various types of blood cells.
Harvesting Hematopoietic Stem and Progenitor Cells
- Mobilization of Stem Cells: Initially, a mobilization agent is administered to the patient. This agent stimulates the movement of hematopoietic stem and progenitor cells from the bone marrow into the bloodstream.
- Apheresis Process: Once in the bloodstream, these cells are collected through a process called apheresis. This process involves drawing blood from the patient, separating out the stem cells, and returning the remaining blood components back to the patient.
What is SCD or sickle cell disease?
In the United States, around 100,000 individuals suffer from sickle cell disease, with approximately 20% experiencing its severe form.
This condition predominantly affects the Black population, with one in every 365 Black infants being born with it.
Scientists have found that carrying the sickle cell trait offers some protection against severe malaria.
Consequently, sickle cell disease is more prevalent in regions with high mosquito activity, like Africa, or among descendants of people from such areas.
See Also; Xromi capsules: uses, dosage, side effects and composition
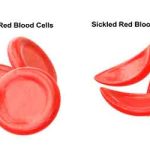
Sickle cell disease impacts hemoglobin, the oxygen-carrying protein in red blood cells.
A genetic alteration leads to the deformation of these cells into a sickle or crescent shape, causing blockages in blood flow. This results in intense pain, potential organ damage, strokes, and other complications.
The main treatment options currently available are medications and blood transfusions.
Bone marrow transplant remains the only definitive cure, but it requires a closely matched donor and carries the risk of rejection.
Sickle Cell Disease (SCD) is a group of inherited red blood cell disorders. It’s characterized by the presence of abnormal hemoglobin, called hemoglobin S (HbS), in red blood cells.
This abnormal hemoglobin causes the red blood cells to become rigid and crescent- or sickle-shaped.
SCD is a complex disease that requires a multifaceted approach for management, including medical treatment, lifestyle adjustments, and psychosocial support.
Ongoing research and advances in treatment are continually improving the outlook for individuals with SCD, aiming towards more effective management and potentially curative therapies.
Genetic Cause of Sickle cell Disease
SCD is caused by a mutation in the gene that codes for the beta chain of hemoglobin.
This mutation leads to the production of abnormal hemoglobin S. SCD is an autosomal recessive condition, meaning a person needs to inherit two copies of the sickle cell gene (one from each parent) to have the disease.
Symptoms and Complications
Sickle-shaped cells can block blood flow in small blood vessels, leading to pain and organ damage, a condition known as a sickle cell crisis or vaso-occlusive crisis.
Other complications include anemia (due to the rapid breakdown of the sickle cells), infections, stroke, and chronic pain.
Vaso-Occlusive Crises
These are episodes of pain that occur when sickled red blood cells obstruct capillaries and restrict blood flow to an organ, resulting in ischemia, pain, and organ damage.
Diagnosis of SCD
SCD is usually diagnosed through a blood test. Newborns are often screened for SCD in many countries as part of routine newborn screening programs.
Treatment of SCD
Treatment of SCD focuses on managing and preventing symptoms and complications.
This may include pain management, hydration, blood transfusions, and the use of medications like hydroxyurea.
Bone marrow or stem cell transplants may offer a cure in some cases, but they are not a viable option for all patients due to various factors including the availability of suitable donors.
Prevalence
SCD is more common in certain ethnic groups, particularly among people with African, Mediterranean, Middle Eastern, and Indian ancestry as earlier mentioned.
Chronic Nature and Management
SCD is a chronic and lifelong condition that can have significant impacts on a person’s quality of life.
Managing SCD typically involves regular healthcare visits, treatments to reduce symptoms and prevent complications, and lifestyle adjustments to maintain health and wellbeing.
Recent Advances
There have been significant advances in the treatment and management of SCD, including the development of new drugs and gene therapy techniques like CRISPR-Cas9, which hold the promise of more effective treatments or even a cure.
Psychosocial Impact
- Quality of Life: Individuals with SCD may face various psychosocial challenges, including coping with chronic pain, frequent hospitalizations, and the impact of the disease on education, employment, and social relationships.
- Mental Health: There is an increased risk of mental health issues, including depression and anxiety, due to the chronic nature of the disease and its complications.
Research and Innovation
- Gene Therapy: Research in gene therapy is promising for SCD. Techniques like CRISPR/Cas9 gene-editing offer potential cures by correcting the genetic mutation at the DNA level.
- New Medications: New pharmacological treatments are being developed that aim to increase the production of fetal hemoglobin, reduce the sickling of red blood cells, or treat specific symptoms and complications of SCD.
Genetic Counseling
Genetic counseling is recommended for individuals with a family history of SCD, especially for prospective parents.
Lifestyle Adjustments
Managing SCD often involves lifestyle adjustments to minimize crises, such as staying hydrated, avoiding extreme temperatures, and managing stress.
Community Support
Support groups and community resources can be invaluable in providing emotional support and practical advice for individuals and families affected by SCD.
Side effects of Casgevy
It’s important for patients receiving Casgevy to be aware of potential side effects, such as:
- Hypersensitivity reactions
- Neutrophil engraftment failure
- Longer median platelet engraftment times
It’s also critical for patients to discuss fertility preservation options with their healthcare provider before treatment, as the conditioning medicine used in the process may impact fertility.
What other drugs will affect Casgevy?
See below;
- Hydroxyurea,
- Crizanlizumab, and
- Voxelotor
- Myeloablative
- Granulocyte-colony stimulating Factor (G-CSF)
No formal drug interaction studies have been performed but Casgevy is not expected to interact with the hepatic cytochrome P-450 enzymes or drug transporters.
Casgevy Administration
The administration of Casgevy involves several steps, starting with mobilization medicine to move blood stem cells from the bone marrow to the bloodstream, followed by apheresis to collect these cells.
The collected cells are then sent to a manufacturing site to create Casgevy, which can take up to six months.
Before the stem cell transplant, the patient receives conditioning medicine to prepare the bone marrow for the modified cells.
Post-infusion, the patient is closely monitored in the hospital for recovery, which can take 4-6 weeks.
Patients should avoid donating blood, organs, tissues, or cells in the future after receiving Casgevy.
Summary of the Administration Process
- Mobilization: Initially, a mobilization medicine is used to move blood stem cells from the bone marrow into the bloodstream.
- Collection (Apheresis): These cells are then collected and sent to a manufacturing facility to create Casgevy.
- Manufacturing: The creation of Casgevy from the patient’s cells can take up to six months.
- Conditioning: Before transplantation, patients undergo conditioning to prepare the bone marrow for the modified cells.
- Infusion: Casgevy is administered via intravenous infusion, after which patients are monitored closely in the hospital.
Casgevy Breakthrough
Casgevy (exagamglogene autotemcel or exa-cel) represents a significant advancement in the treatment of sickle cell disease (SCD), particularly for patients aged 12 years and older who experience recurrent vaso-occlusive crises (VOCs).
Its approval by the FDA as the first gene therapy utilizing CRISPR/Cas9 technology for SCD marks a milestone in modern medicine.
Here’s a more detailed look at various aspects of this groundbreaking treatment:
Mechanism of Action
- CRISPR/Cas9 Technology
Casgevy uses this technology to edit the DNA at specific points, particularly targeting the BCL11A gene.
This gene plays a critical role in the transition from fetal hemoglobin (HbF) to adult hemoglobin (HbA).
By reducing the activity of BCL11A, the therapy promotes continued production of HbF, which is less prone to sickling than the HbA found in patients with SCD.
- Autologous Hematopoietic Stem Cell Transplant (HSCT)
The treatment involves using the patient’s own hematopoietic stem and progenitor cells.
These cells are collected, edited to reduce BCL11A gene activity, and then infused back into the patient.
Efficacy and Safety
- Targeted SCD Treatment: The primary goal of Casgevy is to reduce the frequency of VOCs in SCD patients, which are episodes where sickled red blood cells obstruct blood flow, causing pain and tissue damage.
- Side Effects and Risks: Potential side effects include hypersensitivity reactions, risk of neutrophil engraftment failure, and longer times for platelet engraftment. Patients are monitored for these and other possible complications.
- Long-Term Monitoring: Given the novel nature of this therapy, patients who receive Casgevy are likely to be monitored over a long period to assess long-term safety and efficacy.
Considerations and Precautions
- Impact on Fertility: The conditioning medicine used in the process may affect fertility, necessitating discussions about fertility preservation before treatment.
- Drug Interactions: Patients are advised to inform their healthcare providers about all medications they are taking, as certain drugs may interact with the treatment.
Regulatory Status
- FDA Approval: Casgevy received FDA approval on December 8, 2023, setting a precedent for future gene-editing therapies.
Implications for SCD Treatment
Casgevy’s approval has been hailed as a breakthrough in SCD treatment, offering hope for a more effective management of this chronic and debilitating condition.
Its use of gene-editing technology represents a significant leap forward in the field of genetic medicine, potentially paving the way for similar therapies for other genetic disorders.
Impact on Sickle Cell Disease Treatment
- Novel Approach: Casgevy’s approach, using CRISPR/Cas9 gene-editing technology, is groundbreaking. It represents a shift from symptomatic treatment of SCD to a more fundamental, genetic-level intervention.
- Reducing VOC Incidents: The primary aim is to lessen the occurrence of vaso-occlusive crises, which are painful and can lead to serious complications in SCD patients.
Patient Monitoring and Follow-Up
- Long-Term Observation: Due to the novel nature of this treatment, patients receiving Casgevy require prolonged monitoring to observe long-term effects and potential late-onset side effects.
- Clinical Trials Data: Ongoing and future clinical trials will continue to provide vital information on the efficacy and safety of this treatment, which will be crucial for its broader application in SCD therapy.
Ethical and Social Considerations
- Genetic Editing: The use of gene-editing technology raises ethical questions about genetic intervention, which will likely be a topic of ongoing discussion in the medical community and society at large.
- Accessibility and Cost: As with many advanced therapies, issues related to the accessibility and cost of Casgevy will be important factors in its impact on the broader patient population.
Future Directions
- Potential for Other Diseases: The success of Casgevy could pave the way for similar gene-editing therapies for other genetic disorders.
- Research and Development: Continued research is essential to improve the efficacy, safety, and applicability of gene-editing technologies in medicine.
Regulatory and Medical Community Response
- FDA’s Role: The FDA’s approval of Casgevy underscores the regulatory body’s commitment to advancing innovative therapies for complex diseases.
- Medical Community’s Adaptation: As with any new treatment, the medical community will need to adapt to the inclusion of gene-editing therapies in treatment protocols, which may include specialized training and development of new treatment guidelines.
Patient and Caregiver Education
- Informed Decision-Making: Patients and caregivers must be provided with comprehensive information to make informed decisions about using Casgevy, including understanding potential risks and benefits.
- Support Systems: Adequate support systems, including counseling and patient education programs, will be crucial in managing the physical and psychological aspects of undergoing such a novel treatment.
FAQs about Casgevy
- What is the cost of Casgevy therapy?
The Manufacturer Vertex Pharmaceuticals has stated that Casgevy will be priced at $2.2 million.
- When was Casgevy?
Casgevy was approved by the FDA on December 8, 2023, marking a significant advancement in the treatment of SCD using gene-editing technology.
- What are the potential side effects of Casgevy?
Possible side effects include hypersensitivity reactions, neutrophil engraftment failure, and a risk of bleeding due to longer median platelet engraftment times.
- How is Casgevy administered?
Casgevy is designed for a single administration as part of a hematopoietic stem cell transplant. In this procedure, the patient’s CD34+ cells are genetically altered to lower BCL11A expression in cells of the erythroid lineage. This modification results in an increase in fetal hemoglobin (HbF) production.
- What does CRISPR do in casgevy?
Casgevy involves a treatment where a patient’s own stem cells, altered using CRISPR/Cas9 technology, are infused back into the body to increase fetal hemoglobin production.
This method addresses the issue of sickle-shaped red blood cells, which often lead to severe and painful blockages in blood vessels, known as vaso-occlusive crises (VOC), in various organs.
Concluding remark on what is Casgevy
Casgevy’s approval is a significant milestone in the treatment of sickle cell disease, offering hope for a more effective management of this condition.
However, its long-term impact, both medically and socially, will continue to unfold as more data becomes available and as the therapy becomes more widely used.
Since your opinion counts on our platform feel free to air your view in the comment box.
Recommendations
Drugs used for the treatment of osteoarthritis
Drugs in Sports: Prohibited List and Examples
Organoleptic Evaluation of Herbal Drugs
Standardisation and Evaluation of Herbal Drugs
Methods of Evaluation of Herbal Drugs
Factors Affecting Different Batches of the Same Herbal Ingredient (Crude Drugs)
Microscopic Evaluation of Herbal Drugs
